Page 188 - CW E-Magazine (2-7-2024)
P. 188
Special Report Special Report
Small-molecule drugs: Market, sourcing and supply-chain Germany, Italy, and Spain) and the UK,
69% 59% 72% 75% 79% while the US has contributed less than
trends 5% to global growth. Non-core markets
have experienced stronger growth than
2023: 55 new 2022: 37 new 2021: 50 new 2020: 53 new 2019: 48 new the US, including the Commonwealth of
hat are key trends and Within small molecules, specialty PATRICIA VAN ARNUM drugs approved; 38 drugs approved; 22 drugs approved; 36 drugs approved; 40 drugs approved; 38 Independent States (CIS) with a CAGR
small molecules
small molecules
small molecules
small molecules
small molecules
issues that are on the industry’s small-molecule drugs are growing more Editorial Director, (DCAT) Fig. 2: Percentage of New Drug Approvals by the US Food and Drug Administration’s Center of 11%, India with a CAGR of 10%, and
Wradar impacting sourcing for than twice as fast as those of traditional for Drug Evaluation and Research That Were Small Molecules 2019-2023 Latin America with a CAGR of 9% (see
small-molecule drugs? DCAT Value small-molecule drugs (see Figure 1). How- in 2022, which represented a recent low. Small molecule approvals are new molecular entitires approved via a new drug application (NDA) by Figure 3).
Chain Insights examines market share, ever, traditional small-molecule drugs In 2022, 59% of the new drug approvals the FDA’s center for durg evalution and research; NDA approvals include approvals of drugs and diagnostic
agents.
growth rates, product innovation, and API still dominate the small-molecule sector, by FDA’s CDER were small molecules or Source: US Food and Durg Administrations’s Center for Durg Evaluation and Research Small-molecule API supply lines
supply chains. accounting for 71% of small-molecule 22 of the 37 new drug approvals. Between and 15 new biologics. The 17 new bio- treating amyotrophic lateral sclerosis A key issue potentially impacting
drugs in 2023. Specialty medicines, as 2018 and 2021, small molecules aver- logics approvals in 2023 surpassed 2022 (ALS, i.e., Lou Gehrig’s disease); Glaxo- fi ne chemical producers and CDMOs in
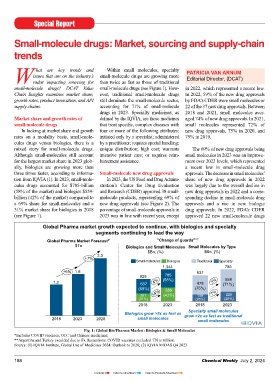
Market share and growth rates of defi ned by the IQVIA, are those medicines aged 74% of new drug approvals. In 2021, levels and matched a recent high in SmithKline’s Jesduvroq (daprodustat) for the Europe Union (EU) relates to policy
small-molecule drugs that treat specifi c, complex diseases with small molecules represented 72% of 2018, when 17 new biologics were also treating anaemia due to chronic kidney moves to increase manufacturing in the
In looking at market share and growth four or more of the following attributes: new drug approvals, 75% in 2020, and approved by FDA’s CDER. The 17 new disease; Novartis’ Fabhalta (iptacopan) EU and reduce dependence on other
rates on a modality basis, small-mole- initiated only by a specialist; administered 79% in 2019. biologic drug approvals in 2023 far for treating paroxysmal nocturnal hemo- countries for active pharmaceutical ingre-
cules drugs versus biologics, there is a by a practitioner; requires special handling; exceeded approvals of new therapeutic globinuria, a rare blood disorder; Novo dients (APIs) and related inputs for critical
mixed story for small-molecule drugs. unique distribution; high cost; warrants The 69% of new drug approvals being biologics by FDA’s CDER of 14 in 2021, Nordisk’s Rivfl oza (nedosiran) for treat- medicines. Earlier this year (April 2024),
Although small-molecules still account intensive patient care; or requires reim- small molecules in 2023 was an improve- 13 in 2020, and 10 in 2019. ing primary hyperoxaluria, a rare condi- the European Commission launched the
for the largest market share in 2023 glob- bursement assistance. ment over 2022 levels, which represented tion characterized by recurrent kidney Critical Medicines Alliance. First an-
ally, biologics are growing more than a recent low in small-molecule drug Product innovation: small-molecules and bladder stones; and Pfi zer’s Paxlovid nounced by the European Commission
three times faster, according to informa- Small-molecule new drug approvals approvals. The decrease in small molecules’ and fi rst-in-class drugs (nirmatrelvir tablets; ritonavir tablets, in October 2023, the Alliance will focus
tion from IQVIA (1). In 2023, small-mole- In 2023, the US Food and Drug Admini- share of new drug approvals in 2022 Aside from just the overall number of co-packaged) for treating COVID-19. on industrial policy and complements the
cules drugs accounted for $785-billion stration’s Center for Drug Evaluation was largely due to the overall decline in new drug approvals, product innovation reform of the EU’s pharmaceutical legis-
(58% of the market) and biologics $559- and Research (CDER) approved 38 small- new drug approvals in 2022 and a corre- can also be evaluated by the number of Although small-molecule drugs were lation as proposed by the European Com-
billion (42% of the market) compared to molecule products, representing 69% of sponding decline in small-molecule drug new drug approvals classifi ed as “fi rst-in- well represented with 85% of the fi rst-in- mission. Set up as a consultative mecha-
a 69% share for small-molecules and a new drug approvals (see Figure 2). The approvals and a rise in new biologic class,” which FDA’s CDER characterizes class new drug approvals in 2023, more nism to policy-makers, the Alliance seeks
31% market share for biologics in 2018 percentage of small-molecule approvals in drug approvals. In 2022, FDA’s CDER as drugs with a different mechanism than half of these drugs were for niche to work to enhance security of supply,
(see Figure 1). 2023 was in line with recent year, except approved 22 new small-molecule drugs of action than existing drugs. In 2023, indications. Of the 17 small-molecule strengthen availability of medicines, and
FDA’s CDER approved 20 new drugs fi rst-in-class drug approvals in 2023, reduce EU supply-chain dependencies.
Global Pharma market growth expected to continue, with biologics and specialty that it characterized as fi rst-in-class, nine, or 53%, were for treating orphan/ The recommendations made by the Alli-
segments continuing to lead the way which represented approximately 36% rare diseases, defi ned as a disease affecting ance will serve as a basis for a multi-year
Global Pharma Market Forecast 1* ‘‘Change of guards” 2** of new drug approvals. 200,000 individuals or less in the US. strategic plan containing milestones and
$Tn Biologics and Small Molecules Small Molecules by Type corresponding deadlines for their imple-
$Bn, (%) $Bn, (%)
2.3 Of these 20 fi rst-in-class new drug Small-molecule generic drugs mentation.
+7% approvals, 17 were small molecules, rep- In turning to the generics market,
Small molecules Biologics Traditional Specialty
1,344 785 resenting 85% of fi rst-in-class new drug the market for small-molecule generic Key factors being analysed include
+6% 1.6 637 approvals in 2023 by FDA’s CDER. Of drugs continues to grow with global sales an over-dependency on a limited number
929 785 these 17 fi rst-in-class, small-molecule reaching $307-billion in 2023, grow- of external suppliers, limited diversifi ca-
558
1.2 637 4% (58%) 478 3% (71%) new drug approvals in 2023, eight were ing at a compound annual growth rate tion possibilities, and limited produc-
(69%) 559 (75%) from large to mid-sized bio/pharma com- (CAGR) of 4% between 2021-2023 (see tion capacities. This will build on the
291 14% (42%) 159 7% 228 panies. These eight drugs were: Astellas’ Figure 3), according to information from European Commission’s vulnerability
(31%) (25%) (29%)
2018 2023 2018 2023 Veozah (fezolinetant) for reducing mod- IQVIA (1). On a regional basis, the US analysis of supply-chain bottlenecks of
Specialty small molecules erate-to-severe vasomotor symptoms due accounts for the largest market share with critical medicines on the Union list of
Biologics grow >3x as fast as grow >2x as fast as traditional to menopause; AstraZeneca’s Truqap 27% of the market, followed by Europe critical medicines. The Union list of
2018 2023 2028 small molecules small molecules (capivasertib) for treating advanced at 22%, and China at 18%. Over the critical medicines, which was published
HR-positive breast cancer; Bausch and past fi ve years (2018-2023), Europe as a by the European Medicines Agency last
Fig. 1: Global Bio/Pharma Market: Biologics & Small Molecules Lomb’s Miebo (perfl uorohexyloctane whole contributed almost 30% of gene- December (December 2023), refers to
*Includes COVID vaccines, OTC and Chinese medicines;
**Argentina and Turkey exculded due to Fx fl uctuations. COVID vaccines excluded TN is trillion. ophthalmic solution) for treating dry-eye rics market growth, which includes 18% a list of critical medicines for the EU/
Source: (1) IQVIA Institute, Global Use of Medicines 2024: Outlook to 2028; (2) IQVIA MIDAS Q4 2023 disease; Biogen’s Qalsody (tofersen) for combined growth in the EU4 (France, European Economic Area, which con-
188 Chemical Weekly July 2, 2024 Chemical Weekly July 2, 2024 189
Contents Index to Advertisers Index to Products Advertised