Page 134 - CW E-Magazine (3-9-2024)
P. 134
Point of View
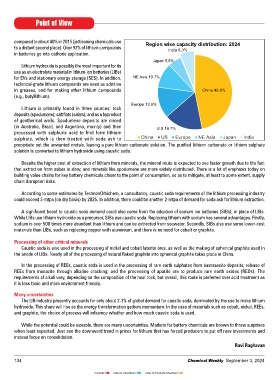
compared to about 40% in 2015 (jettisoning chemicals use Region wise capacity distribution: 2024
to a distant second place). Over 92% of lithium compounds India 6.3%
in batteries go into cathode application.
Japan 5.8%
Lithium hydroxide is possibly the most important for its
use as an electrolyte material in lithium-ion batteries (LIBs)
for EVs and stationary energy storage (SES). In addition, NE Asia 10.7%
technical-grade lithium compounds are used as additive
in greases, and for making other lithium compounds China 46.8%
(e.g., butyllithium).
Europe 13.5%
Lithium is primarily found in three sources: rock
deposits (spodumene); salt flats (salars); and as a byproduct
of geothermal wells. Spodumene deposits are mined
(in Australia, Brazil, and Argentina, mainly) and then U.S 16.7%
processed with sulphuric acid to first form lithium
sulphate, which is then treated with soda ash to China US Europe NE Asia Japan India
precipitate out the unwanted metals, leaving a pure lithium carbonate solution. The purified lithium carbonate or lithium sulphate
solution is converted to lithium hydroxide using caustic soda.
Despite the higher cost of extraction of lithium from minerals, the mineral route is expected to see faster growth due to the fact
that extraction from salars is slow, and minerals like spodumene are more widely distributed. There is a lot of emphasis today on
building value chains for key battery chemicals closer to the point of consumption, so as to mitigate, at least to some extent, supply
chain disruption risks.
According to some estimates by TechnonOrbichem, a consultancy, caustic soda requirements of the lithium processing industry
could exceed 3-mtpa (on dry basis) by 2025. In addition, there could be another 2-mtpa of demand for soda ash for lithium extraction.
A significant boost to caustic soda demand could also come from the adoption of sodium ion batteries (SIBs), in place of LIBs.
While LIBs use lithium hydroxide as a precursor, SIBs use caustic soda. Replacing lithium with sodium has several advantages. Firstly,
sodium is over 500 times more abundant than lithium and can be extracted from seawater. Secondly, SIBs also use some lower-cost
materials than LIBs, such as replacing copper with aluminium, and there is no need for cobalt or graphite.
Processing of other critical minerals
Caustic soda is also used in the processing of nickel and cobalt laterite ores, as well as the making of spherical graphite used in
the anode of LIBs. Nearly all of the processing of natural flaked graphite into spherical graphite takes place in China.
In the processing of REEs, caustic soda is used in the processing of rare earth sulphates from bastnaesite deposits; release of
REEs from monazite through alkaline cracking; and the processing of apatite ore to produce rare earth oxides (REOs). The
requirements of alkali vary, depending on the composition of the host rock, but overall, this route is preferred over acid treatment as
it is less toxic and more environment-friendly.
Many uncertainties
The LIB industry presently accounts for only about 2-3% of global demand for caustic soda, dominated by the use to make lithium
hydroxide. This share will rise as the energy transformation gathers momentum. In the case of materials such as cobalt, nickel, REEs,
and graphite, the choice of process will influence whether and how much caustic soda is used.
While the potential could be sizeable, there are many uncertainties. Markets for battery chemicals are known to throw surprises
when least expected. Just see the downward trend in prices for lithium that has forced producers to put off new investments and
instead focus on consolidation.
Ravi Raghavan
134 Chemical Weekly September 3, 2024
Contents Index to Advertisers Index to Products Advertised