Page 163 - CW E-Magazine (5-3-2024)
P. 163
Special Report Special Report
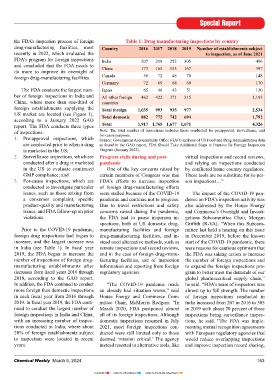
FDA’s inspections of foreign drug manufacturing the FDA’s inspection process of foreign Country Table 1: Drug manufacturing inspections by country
drug-manufacturing facilities, most
2016
2017
2019
Number of establishments subject
2018
facilities recently in 2022, which evaluated the India 207 219 252 305 to inspection, as of June 2021
FDA’s program for foreign inspections
496
and concluded that the FDA needs to
he FDA’s inspection process for 3. The implications of using alter- PATRICIA VAN ARNUM do more to improve its oversight of China 173 165 153 167 397
foreign drug-manufacturing faci- native tools, such as remote in- Editorial Director foreign drug-manufacturing facilities. Canada 56 72 48 70 148
Tlities came under scrutiny once spections, initiated during the Drug, Chemical & Associated Germany 72 69 68 69 170
again with Congress holding a hearing COVID-19 pandemic, instead of Technologies Association,Inc. (DCAT) The FDA conducts the largest num- Japan 65 46 43 51 130
questioning the frequency and qual- in-person inspections to oversee ber of foreign inspections in India and
ity of FDA’s foreign drug inspections. drugs made overseas; pointed to the differences in the fre- China, where more than one-third of All other foreign 462 422 371 315 1,193
countries
What may be next? 4. The frequency and quality of the quency of inspections of foreign drug- foreign establishments supplying the
FDA’s foreign drug inspections manufacturing facilities compared to US market are located (see Figure 1), Total foreign 1,035 993 935 977 2,534
FDA’s foreign drug inspections compared with those of domestic domestic facilities. “What makes all according to a January 2022 GAO Total domestic 882 772 742 694 1,792
program under scrutiny inspections; and of this even more disturbing is that in report. The FDA conducts three types Total 1,917 1,765 1,677 1,671 4,326
The Oversight and Investigations 5. Ways in which the FDA can practice we hold domestic manufactu- of inspections: Note: The total number of inspections includes those conducted for preapproval, surveillance, and
Subcommittee of the Energy and strengthen its foreign drug inspec- rers to much higher standards than we 1. Pre-approval inspections, which for-cause purposes.
Commerce Committee of the US House tion program. do foreign manufacturers….“We need are conducted prior to when a drug Source: Government Accountability Offi ce (GAO) analysis of US Food and Drug Administration data
as found in the GAO report, FDA Should Take Additional Steps to Improve Its Foreign Inspection
of Representatives held a hearing on a level playing fi eld that encourages is marketed in the US; Program (January 2022).
February 6, 2024 to discuss oversight “I’m concerned that the FDA is fail- domestic manufacturing.” 2. Surveillance inspections, which are Progress stalls during and post- virtual inspections and record reviews,
of the US Food and Drug Administra- ing in its mission. It is not adequately conducted after a drug is marketed pandemic and relying on inspections conducted
tion’s (FDA) foreign drug inspection executing its foreign inspection pro- Also testifying at the hearing was in the US to evaluate continued One of the key concerns raised by by confl icted home country regulators.
program. The hearing sought to discuss gram, which was questionable before Mary Denigan-Macauley, Director of GMP compliance; and certain members of Congress was that These tools are no substitute for in-per-
several key issues: the pandemic, became non-existent Health at the US Government Account- 3. For-cause inspections, which are FDA’s efforts to increase inspection son inspections…”
1. The current status of FDA’s during the pandemic, and has seen ability Offi ce (GAO), a legislative conducted to investigate particular of foreign drug-manufacturing efforts
foreign drug inspection program; little improvement since,” said House branch government agency that pro- issues, such as those arising from were stalled because of the COVID-19 The impact of the COVID-19 pan-
2. The challenges that FDA inspec- Energy and Commerce Committee Chair, vides auditing, evaluation, and investi- a consumer complaint, specifi c pandemic and continue not to progress. demic on FDA’s inspection activity was
tors face when conducting foreign Cathy McMorris Rodgers (R-WA) in gative services for the US Congress. product-quality and manufacturing Due to travel restrictions and safety also addressed by the House Energy
drug inspections; comments made at the hearing. She also The GAO has issued several reports on issues, and FDA follow-up on prior concerns raised during the pandemic, and Commerce’s Oversight and Investi-
violations. the FDA had to pause in-person in- gations Subcommittee Chair, Morgan
spections, both at US domestic drug- Griffi th (R-VA). “When this Subcom-
Prior to the COVID-19 pandemic, manufacturing facilities and foreign mittee last held a hearing on this issue
foreign drug inspections had begun to drug-manufacturing facilities, and in- in December 2019, before the known
increase, and the largest increase was stead used alternative methods, such as start of the COVID-19 pandemic, there
in India (see Table 1). In fi scal year remote inspections and record reviews, were reasons for cautious optimism that
2019, the FDA began to increase the and in the case of foreign drug-manu- the FDA was taking action to increase
number of inspections of foreign drug- facturing facilities, use of inspection the number of foreign inspections and
manufacturing establishments after information and reporting from foreign to expand the foreign inspections pro-
decreases from fi scal years 2016 through regulatory agencies. gram to better meet the demands of our
2018, according to the GAO report. global pharmaceutical supply chain,”
In addition, the FDA continued to conduct “The COVID-19 pandemic made he said. “FDA’s team of inspectors was
more foreign than domestic inspections an already bad situation worse,” said almost up to full strength. The number
in each fi scal year from 2016 through House Energy and Commerce Com- of foreign inspections conducted in
2019. In fi scal year 2019, the FDA conti- mittee Chair, McMorris Rodgers. “In India increased from 207 in 2016 to 305
nued to conduct the largest number of March 2020, FDA postponed almost in 2019 with about 70 percent of those
foreign inspections in India and China, all of its foreign inspections. Although inspections being surveillance inspec-
Fig.1: The 10 Countries with the Most Foreign Drug Establishments Manufacturing Drugs for the US Market as of June 2021.
with an increasing number of inspec- domestic inspections resumed in July tions, he said. “The FDA was imple-
Note: This fi gure includes the 10 countries with the most foreign drug establishments manufacturing drugs for the US market and does not include those tions conducted in India, where about 2021, most foreign inspections con- menting mutual recognition agreements
countries with fewer than 75 establishments. The count of foreign establishment does not include approximately 700 foreign establishments that are only
manufacturing alcohol-based hand sanitizers. 20% of foreign establishments subject ducted were still limited only to those with European regulatory agencies that
Source: Government Accountability offi ce (GAO) analysis of US Foof and Drug Administration data and National Atlas (base map) as found in the GAO to inspection were located in recent deemed ‘mission critical.’ The agency would reduce overlapping inspections
report, FDA Should Take Additional Steps to improve its Foreign Inspection Program (January 2022). years. instead resorted to alternative tools, like and improve inspection record sharing,
162 Chemical Weekly March 5, 2024 Chemical Weekly March 5, 2024 163
Contents Index to Advertisers Index to Products Advertised