Page 185 - CW E-Magazine (1-4-2025)
P. 185
Special Report
hydrogen to diffuse out of the metal. matter and are extracted alongside the
If baking is not an option, then use of oil and/or natural gas. These gases
lower strength steels and reducing the readily dissolve in water, lower the
residual and applied stress are possible pH by forming carbonic and sulphuric
ways. These may be the best options acids, and can cause significant corrosion
for circumstances that result in hydro- or cracking of carbon steel – a common
gen absorption while a component is pipeline material of construction. Fig. 7
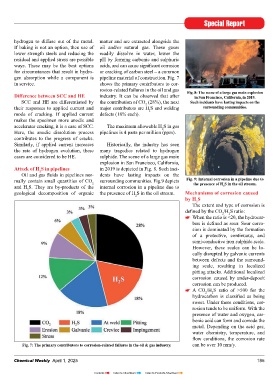
in service. shows the primary contributors to cor-
rosion-related failures in the oil and gas Fig. 8: the scene of a large gas main explosion
difference between Scc and HE industry. It can be observed that after in San Francisco, california, in 2019.
SCC and HE are differentiated by the contribution of CO (28%), the next Such incidents have lasting impacts on the
2
their responses to applied current and major contributors are H S and welding surrounding communities.
2
mode of cracking. If applied current defects (18% each).
makes the specimen more anodic and
accelerates cracking, it is a case of SCC. The maximum allowable H S in gas
2
Here, the anodic dissolution process pipelines is 4 parts per million (ppm).
contributes to the progress of cracks.
Similarly, if applied current increases Historically, the industry has seen
the rate of hydrogen evolution, these many tragedies related to hydrogen
cases are considered to be HE. sulphide. The scene of a large gas main
explosion in San Francisco, California,
Attack of H S in pipelines in 2019 is depicted in Fig. 8. Such inci-
2
Oil and gas fluids in pipelines nor- dents have lasting impacts on the
mally contain small quantities of CO 2 surrounding communities. Fig.9 depicts Fig. 9: Internal corrosion in a pipeline due to
the presence of H S in the oil stream.
and H S. They are by-products of the internal corrosion in a pipeline due to 2
2
geological decomposition of organic the presence of H S in the oil stream. Mechanisms of corrosion caused
2
by H S
2
The extent and type of corrosion is
defined by the CO /H S ratio:
2
2
When the ratio is <20, the hydrocar-
bon is defined as sour. Sour corro-
sion is dominated by the formation
of a protective, continuous, and
semi-conductive iron sulphide scale.
However, these scales can be lo-
cally disrupted by galvanic currents
between defects and the surround-
ing scale, resulting in localized
pitting attacks. Additional localized
corrosion caused by under-deposit
corrosion can be produced.
A CO /H S ratio of >500 for the
2
2
hydrocarbon is classified as being
sweet. Under these conditions, cor-
rosion tends to be uniform. With the
presence of water and oxygen, car-
bonic acid can form and corrode the
metal. Depending on the acid gas,
water chemistry, temperature, and
flow conditions, the corrosion rate
Fig. 7: the primary contributors to corrosion-related failures in the oil & gas industry. can be over 10 mm/y.
Chemical Weekly April 1, 2025 185
Contents Index to Advertisers Index to Products Advertised