Page 134 - CW E-Magazine (23-4-2024)
P. 134
Top Stories Pharmaceuticals
INDUSTRY TRENDS REGULATORY ACTION
India adds record 18-GW renewable energy capacity Natco Pharma’s Telangana plant gets USFDA
in FY2023-24 warning
India has added a record renewable Natco Pharma said it has received a “We wish to inform you that the The drug fi rm will respond to the
energy capacity of 18.48-GW in 2023-24, warning letter from the US health regu- company has received a warning letter letter within the stipulated timelines
which is over 21 percent higher than lator for its Telangana-based manufac- dated April 8, 2024 from the USFDA,” and work closely with the USFDA to
15.27-GW a year ago, according to the turing plant. The US Food and Drug the drug fi rm said in a regulatory fi ling. address the concerns in a holistic and
latest data of the Ministry of New & Administration (USFDA) issued eight The company does not believe that the timely manner to ensure sustained
Renewable Energy. However, indus- observations under Form 483 after warning letter will have an impact on compliance, the company informed.
try experts said there is a need to add inspecting the company’s Kothur- supplies or the existing revenues from A warning letter is issued when the US
at least 50-GW of renewable energy based formulation facility. The inspec- this facility It may cause delay/with- health regulator fi nds that a manufac-
capacity annually for the next six years tion was conducted by USFDA from holding of pending product approvals turer has signifi cantly violated its regu-
to meet the ambitious target of 500-GW October 9- 18, last year. from this site,” it added. lations.
of renewables by 2030.
PRODUCT LAUNCH
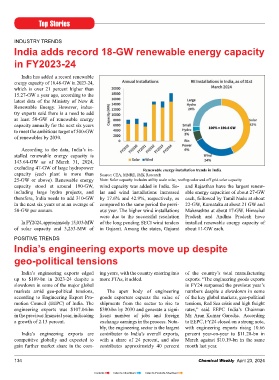
According to the data, India’s in-
stalled renewable energy capacity is Dr. Reddy’s launches migraine management wearable
143.64-GW as of March 31, 2024,
excluding 47-GW of large hydropower Renewable energy installation trends in India device in Germany
capacity (each plant is more than Source: CEA, MNRE, JMK Research
25-GW or above). Renewable energy Note: Solar capacity includes utility scale solar, rooftop solar and off grid solar capacity Dr. Reddy’s Laboratories has certifi ed in Europe. The company is pill burden in migraine,” said Mr. M. V.
capacity stood at around 190-GW, wind capacity was added in India. So- and Rajasthan have the largest renew- launched the drug-free, non-invasive also planning to launch Nerivio in Ramana, CEO, Branded Markets (India
including large hydro projects, and lar and wind installations increased able energy capacities of about 27-GW migraine management wearable device, Spain and the UK. and Emerging Markets), Dr. Reddy’s.
therefore, India needs to add 310-GW by 17.6% and 42.9%, respectively, as each, followed by Tamil Nadu at about Nerivio, in Germany through its step-
in the next six years or at an average of compared to the same period the previ- 22-GW, Karnataka at about 21 GW and down subsidiary, Betapharm. “Nerivio has had an encouraging Last year, Dr. Reddy’s entered into
50-GW per annum. ous year. The higher wind installations Maharashtra at about 17-GW. Himachal start in India, with recommendations an exclusive agreement with Theranica,
were due to the successful resolution Pradesh and Andhra Pradesh have The launch marks the company’s from neurologists in India and bringing a prescribed digital therapeutics com-
In FY2024, approximately 15,033-MW of the long pending SECI wind tenders installed renewable energy capacity of entry into digital therapeutics in relief to patients living with migraine. pany developing advanced neuromodu-
of solar capacity and 3,253-MW of in Gujarat. Among the states, Gujarat about 11-GW each. Europe. Nerivio is approved by the We believe this product meets a genu- lation devices for migraine and other
United States Food and Drug Admini- ine unmet clinical need among migraine pain conditions, to distribute and market
POSITIVE TRENDS stration (USFDA) and is CE-mark patients, and has the potential to reduce Nerivio in multiple markets.
India’s engineering exports move up despite USFDA fi nds manufacturing lapses at Kilitch
geo-political tensions Healthcare’s Navi Mumbai plant
India’s engineering exports edged ing years, with the country entering into of the country’s total manufacturing
up to $109-bn in 2023-24 despite a more FTAs, it added. exports. “The engineering goods exports The US health regulator has pulled The warning letter summarises state of disrepair, poorly cleaned and
slowdown in some of the major global in FY24 surpassed the previous year’s up Kilitch Healthcare India for manu- signifi cant violations of current good maintained,” the letter stated. A warning
markets amid geo-political tensions, The apex body of engineering numbers despite a slowdown in some facturing lapses, including packaging manufacturing practice (cGMP) regu- letter is issued when the US health
according to Engineering Export Pro- goods exporters expects the value of of the key global markets, geo-political drugs in insanitary conditions at its lations for fi nished pharmaceuticals, regulator fi nds that a manufacturer has
motion Council (EEPC) of India. The shipments from the sector to rise to tensions, Red Sea crisis and high freight Maharashtra-based plant. the US health regulator said. signifi cantly violated its regulations.
engineering exports was $107.04-bn $300-bn by 2030 and generate a signi- rates,” said EEPC India’s Chairman
in the previous fi nancial year, indicating fi cant number of jobs and foreign Mr. Arun Kumar Garodia. According In a warning letter to the company’s “Because your drug products were The USFDA inspected the plant on
a growth of 2.13 percent. exchange earnings in the process. Nota- to EEPC, FY24 closed on a strong note, Managing Director Mr. Paresh Mehta, prepared, packed, or held under insani- October 12-20, 2023. The regulator
bly, the engineering sector is the largest with engineering exports rising 10.66 the US Food and Drug Administration tary conditions, whereby they may also asked the company to conduct
India’s engineering exports are contributor to India’s overall exports, percent year-on-year to $11.28-bn in (USFDA) pointed out various lapses at have become contaminated with fi lth or a comprehensive investigation into
competitive globally and expected to with a share of 24 percent, and also March against $10.19-bn in the same the Navi Mumbai plant, which produc- rendered injurious to health. FDA investi- the extent of the inaccuracies in data
gain further market share in the com- contributes approximately 40 percent month last year. es fi nished pharmaceuticals. gators observed your facility to be in a records and reporting.
134 Chemical Weekly April 23, 2024 Chemical Weekly April 23, 2024 135
Contents Index to Advertisers Index to Products Advertised